Periodic Table of Elements Easy to Read
Periodic Table of Elements
Only jump to your interested topics from below.

#1 What is a classification? And why we need nomenclature?
#2 Brief history of periodic table
#three How are elements arranged in periodic table?
#four Rows and columns in periodic table
#v Basics of periodic table
#vi How to read periodic table elements?
#seven What does group number and period number represent on the periodic table?
#8 Blocks in periodic table
#nine Trends in periodic tabular array (Periodic trends)
What is a classification? And why nosotros demand nomenclature?

What if someone asks you to find out one item volume from this huge stack of books.
Will you be able to find that item book easily?
The answer is: NO

Look at this epitome, you lot can see that all the books are arranged in a particular shelf co-ordinate to their similarities.
(In other words, books are grouped or sorted according to their similarities.)
At present, what if yous have to notice a particular book from this properly arranged shelf?

It becomes easy for you to find that particular book, right?
The same thing is with elements.
There are full 118 elements discovered till now.
These elements also need to be classified or grouped together co-ordinate to their similarities.
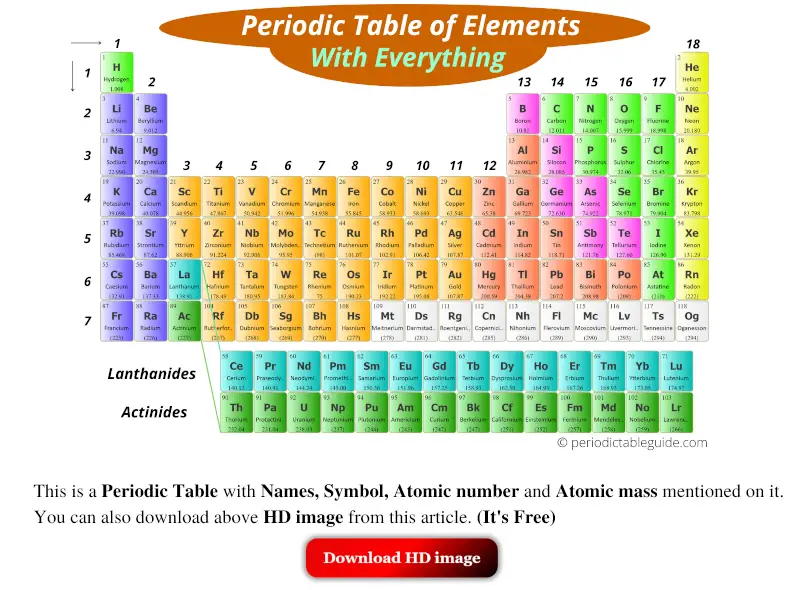
Now, this table looks amazing and well classified. Isn't information technology?
Well, allow me come to the main point.
Why classification of elements is needed?

A big question. Why do we demand to classify all the elements?
All the elements take their ain physical and chemical properties.
These elements take dissimilar uses, applications, chemical reactions, atomic construction, electron configuration, etc.
Thus information technology is necessary to classify (or group or sort) all these 118 elements according to their similarities.
After proper classification of elements, it becomes very easy for anyone to find a particular chemical element from the table.
Also, the report of each and every chemical element becomes easy by referring to the well classified elements.
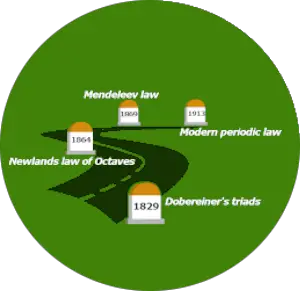
Brief history of periodic table
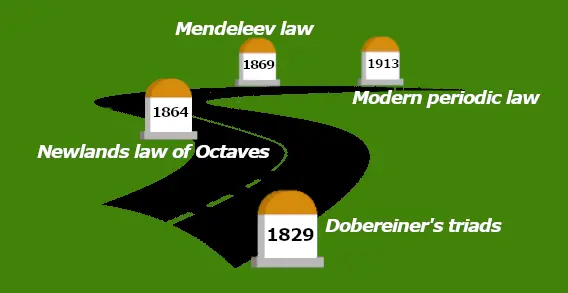
This motion picture shows you how the Periodic Tabular array evolved.
History of Periodic tabular array:
| Discovery | Year |
| Dobereiner'due south triads | 1829 |
| Newlands police of octaves | 1864 |
| Mendeleev police | 1869 |
| The modern periodic constabulary | 1913 |
Permit me requite you a brief introduction about the same.
1). Dobereiner's triads (1829)
Johann Wolfgang Dobereiner (A person backside the discovery of triads)
But await. What are triads?

Here is a meaning hidden in the proper name itself.
Tri means 3
During the time of Dobereiner, around 30+ elements were discovered.
Dobereiner arranged few of these known elements in the grouping of three elements.
Let me bear witness you with an example.
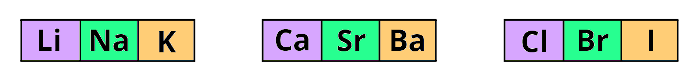
He arranged;
lithium (Li), sodium (Na) and potassium (One thousand) in i triad (group of 3 elements),
Calcium (Ca), strontium (Sr) and Barium (Ba) in other triad and,
Chlorine (Cl), bromine (Br) and iodine (I) in other triad.
The main thing I want to tell y'all is that he arranged these elements in the increasing club of their Atomic MASS.
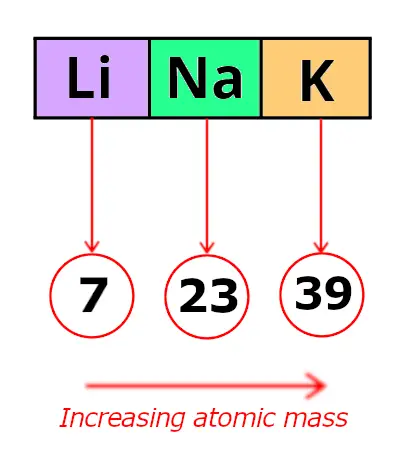
Now, let me recall to yous the atomic mass of lithium, sodium and potassium.
Triad 1
| Element | Atomic mass |
| Lithium (Li) | seven |
| Sodium (Na) | 23 |
| Potassium (Yard) | 39 |
In these triads, Dobereiner found that the atomic mass of the heart element is the average of the diminutive mass of the outset and third chemical element.
I'll explain.
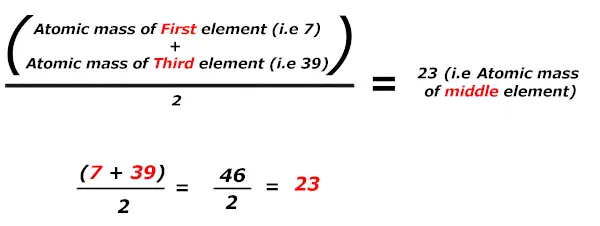
Kudos, large magic !!
Same way this similarity was seen for 2nd triad as well as 3rd triad.
Statement of Dobereiner's triads:
When elements are arranged in increasing order of their atomic mass, in grouping of three (triads), then the arithmetic mean of mass of 1st and 3rd element is found to exist approximately equal to the mass of central element.
He also stated that the elements of the same triad show similar properties. (Means Lithium, sodium and potassium are in same triad and so they shows similar properties)
Limitations of Dobereiner's triads
- Dobereiner's triads were applicable for but few elements. It was not applicable for all the 30+ known elements.
Hope you accept understood this, now let's motion to the next discovery.
two). Newland'south law of octaves (1864)

A human backside the discovery of Newland's law of octaves: John Newlands
A bones question. What is octave?

October indicates the 8
(Note: It is non Oct, but if you know about the IUPAC classification, and then you lot might have heard that oct is used to stand for viii)
John Newlands discovered his law of octaves in the year 1864.
During this fourth dimension, 56 elements were discovered.He arranged these known elements in the increasing order of their ATOMIC MASS.
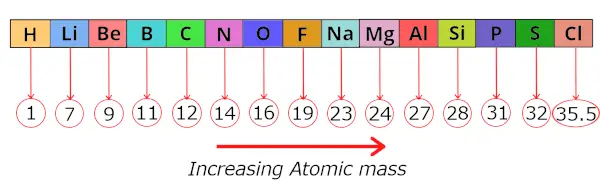
He institute that after this arrangement, every 8th element shows a similar property as that of its respective 1st elements.
Let me give you an example.
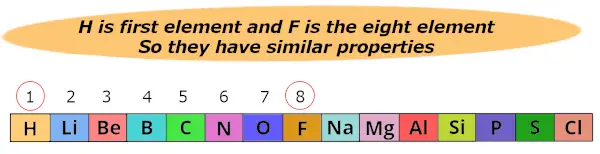
Here hydrogen (H) is 1st element and Fluorine (F) is the 8th element.
Then Newlands found that these 2 elements show similar properties.
Another example;
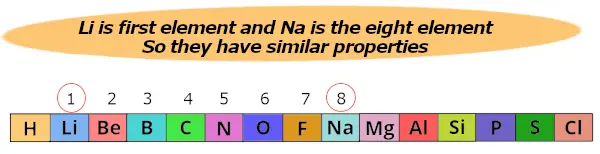
Hither lithium (Li) is 1st element and Sodium (Na) is the 8th element.
And so both these elements prove similar backdrop.
Thus, Newlands constitute that if all the elements are arranged in increasing order of their atomic mass, then every 8 elements shows the like property.
Hence, the proper noun of this police is Newland's law of octaves.
Statement of Newland's law:
If elements are bundled in the increasing order of their diminutive mass, then the property of every eight elements (starting from the first element) repeats.
Limitations of Newlands law of octaves
- Similarity in properties as per the law was seen up to calcium simply.
- He stated that only 56 elements exist in nature. But afterward, he was proved incorrect and many more than elements were discovered.

- Dissimilar elements were placed in the same slot.
- Similar elements were placed in different slots.
iii). Mendeleev law (1869)

Wow, what a personality !!!
Amazing.
Mendeleev law:
The properties of elements are the periodic functions of theirs atomic masses
Dmitri Ivanovich Mendeleev discovered this law and it is known as Mendeleev constabulary.
Well, allow me explicate this to you with a simple story.
It'due south a story of 1869.
During that fourth dimension, 63 elements were known.
Mandeleev prepared the cards (just like the solitaire cards) of all these 63 elements with their private properties written on each card.
He took a board and and then he arranged all these 63 elements in the increasing club of their atomic mass.
First he took the element with lowest atomic mass, and started placing other elements besides it.
If the property of any of the elements were similar, then he used to place information technology in the aforementioned column. And if the property of elements did not match, so he kept it in the row.
That way he filled the entire table and finally the table was prepared equally shown below.
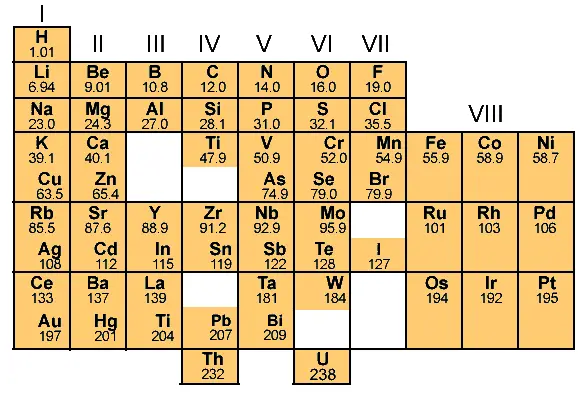
This table is known as the Mendeleev periodic table of elements.
At that place are a total 8 vertical columns in the Mendeleev periodic table, which are known equally groups.
And there are 7 horizontal rows, which are known as periods.
But wait.
Take you lot noticed that many blocks have more than i element in Mendeleev periodic table? Why?
The short answer: Those elements prove similarly in their properties. That's why Mendeleev placed those elements in the same block.
One more question.
Why are some blocks empty in the above Mendeleev periodic table?
Now look, while arranging the elements according to atomic masses, Mendeleev found that the properties of few elements were not matching with whatever of the previous ones, so he didn't place such elements in that particular column.
And then there were some empty blocks seen on the Mendeleev periodic tabular array.
Understood?
I know this seems a little difficult for you, so you can refer to this detailed guide on "Mendeleev periodic table" where I accept explained all these things with proper images for your amend understanding. I have also discussed the merits and demerits of the Mendeleev periodic table. And then must visit that article.
4). Modern Periodic Law (1913)
We have seen in previous department that Dobereiner'due south law, Newland's law every bit well as Mendeleev law were based on the arrangements of elements according to their increasing diminutive masses.
Merely the simply difference in modern periodic law is;

Yes, all the elements in modernistic periodic table are arranged on the basis of their Atomic NUMBER.
Modern periodic law:
The properties of the elements are the Periodic function of their Diminutive NUMBERS.
Modern Periodic Law was given by Henry Moseley in 1913.
This police is exactly like to the Mendeleev law, just the merely difference is;
- Mendeleev arranged the elements according to the increasing diminutive mass. While,
- Henry Moseley bundled the elements according to the increasing diminutive number.
In other words;
- Mandeleev law says that the backdrop of elements are the functions of their atomic mass. While,
- Modernistic periodic law says that the properties of elements are the Periodic role of their diminutive number.
According to the modern periodic police force given by Henry Moseley, the elements should be arranged in the tabular array on the basis of their Diminutive NUMBER.
That'southward it!
Afterward arranging all the elements based on atomic number, the modern periodic table came into existence.
How are elements bundled in periodic table?
Here, yous will exactly come up to know how Henry Moseley arranged the elements in periodic table on the basis of modernistic periodic law.
Every bit said in his modernistic periodic constabulary, the elements should exist arranged according to the increasing atomic number.
So the minimum diminutive number (i.due east ane) was placed at the left-top corner of the table.
Further, the elements with atomic number ii, 3, four, v, 6, etc… were placed in the blocks of periodic table.
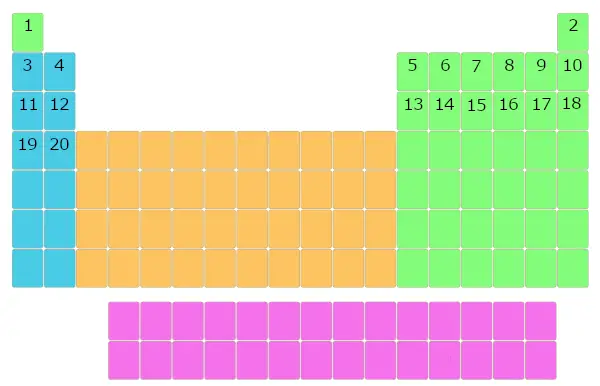
Similarly, all the elements were filled in this table of elements.
But, here is an of import thing to know.
See the to a higher place epitome. The actual periodic tabular array is like this. This Periodic table is besides known equally a long form of periodic table.
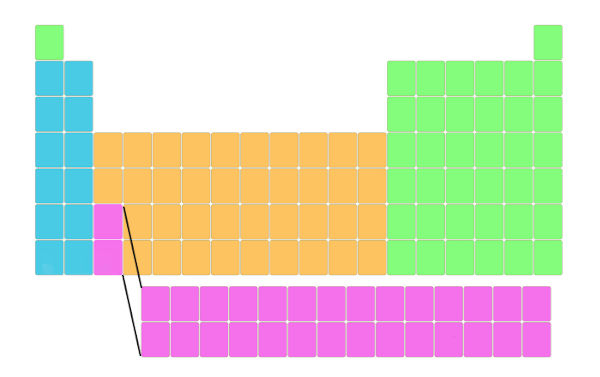
Simply it is represented similar this then that it fits perfectly and it will non distort the shape of the periodic table. Also the elements lying in this block have different electron configuration and dissimilar properties. Well, do not worry about this, I'll explain to yous well-nigh the electronic configuration and properties later.
All the same confused?
Expect, the element having diminutive number 57 is here every bit shown in the below pic.
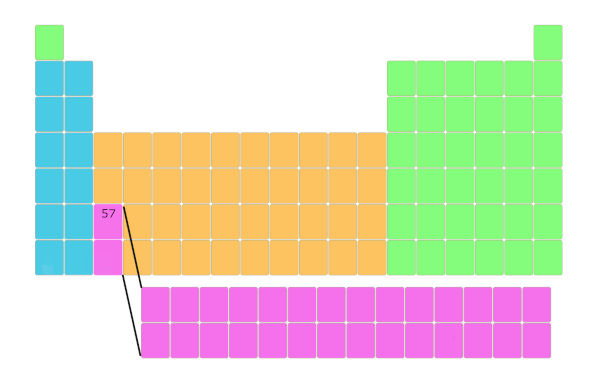
Now, element 58 is non placed likewise it, but it is placed in a carve up cake as shown below. And this repeats upwards to element number 71.
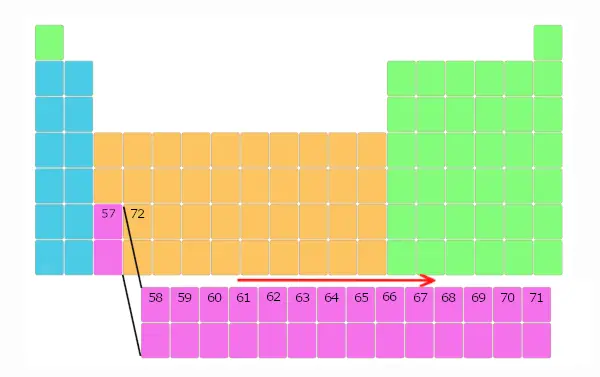
Thus, elements with atomic number 58 to 71 are placed in split cake below the main table.
Similarly, elements with diminutive number 90 to 103 are also placed in separate block equally shown beneath.
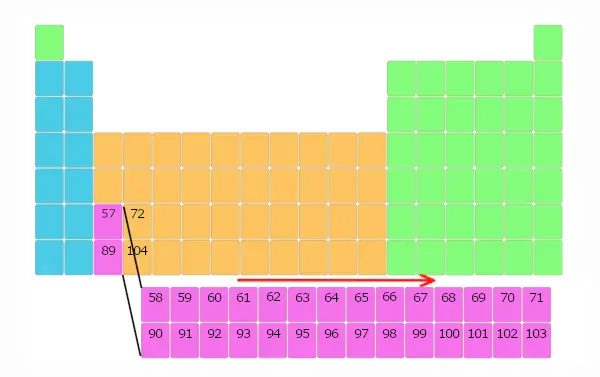
Just await for few seconds, Y'all volition definitely come to know the reason why these elements are placed in a separate block.
Keep reading…
Rows and columns in periodic table
I desire to explicate you the basics of periodic tabular array, just earlier that, let us continue with the rows and columns of the Periodic table.
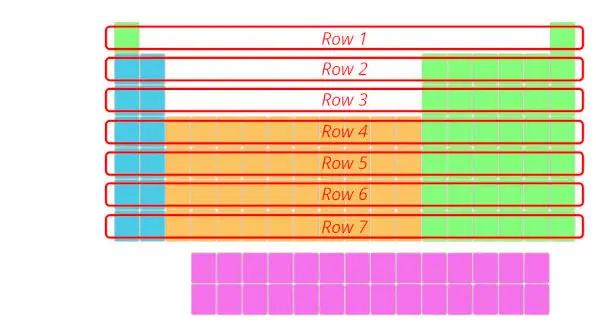
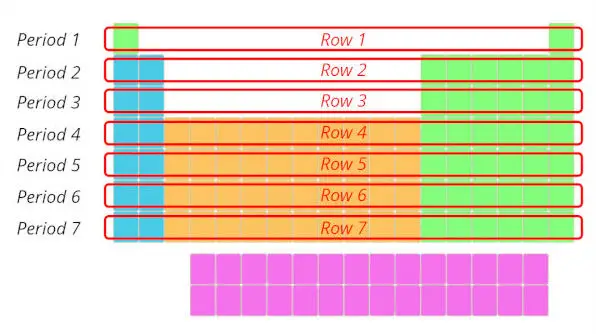
1). Rows in periodic tabular array (Periods)
There are total 7 rows in periodic table.
Question: Y'all might be having a question that "What are Periods in Periodic table?"
Answer: The rows in Periodic table are known equally Periods.
The first row is known as 1st catamenia, 2nd row is known as 2nd period and similarly you tin can run into in the below paradigm till the 7th row.
Now, allow's move to the columns of the Periodic tabular array.
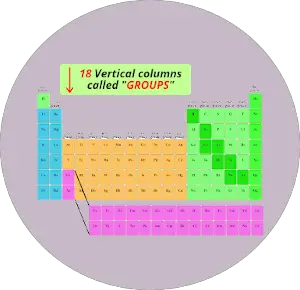
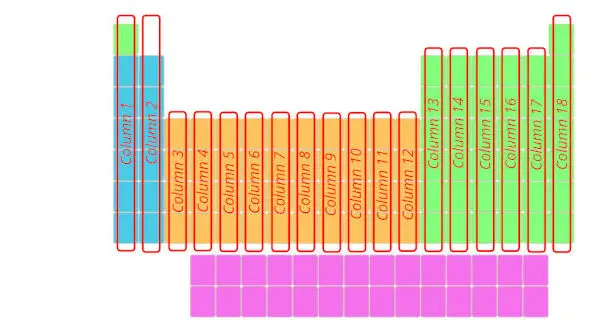
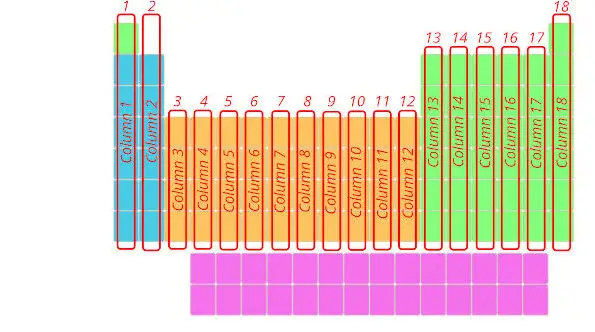
2). Columns in periodic table (Groups)
Merely see below, there are total 18 columns in the Periodic table.
If you are having a question "What are Groups in Periodic table?", then here is a short and sweetness answer for you.
Reply: The columns in Periodic table are known as Groups.
The first cavalcade is known as the 1st group, second column is known as 2nd group and similarly you tin see in the below image till the 18th cavalcade.
Now, information technology'due south time to run into the entire periodic table. Hurray!!!
Nuts of periodic table
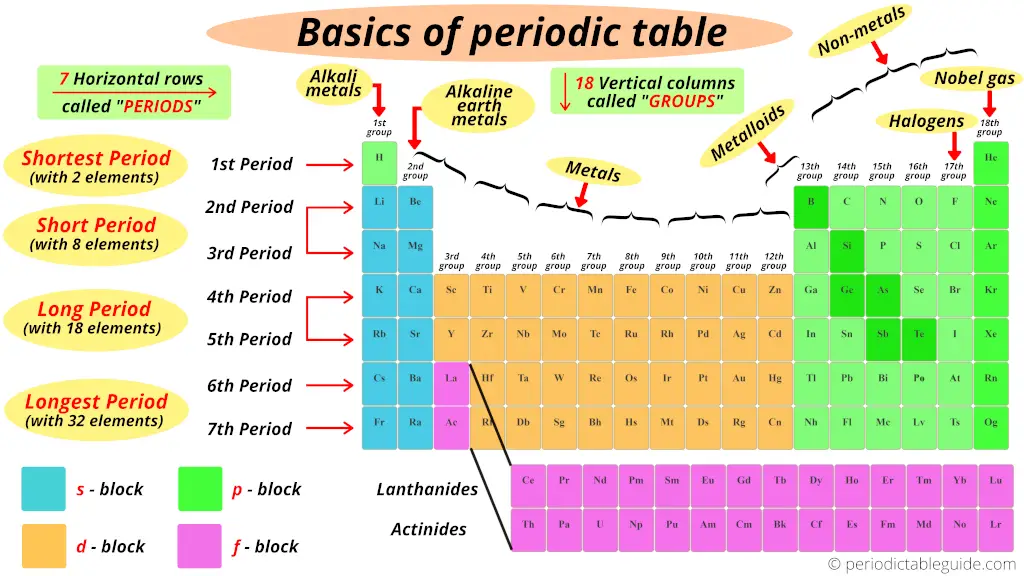
In this section you volition come to know all the basis things related to the modern periodic table like;
- Groups and periods of periodic table
- Blocks of Periodic table
- Types of Elements on periodic table
- How to read periodic table?, etc..
1). Groups and periods of periodic table
Nosotros have already seen in the above section that the vertical columns in the modernistic periodic table are known equally groups.
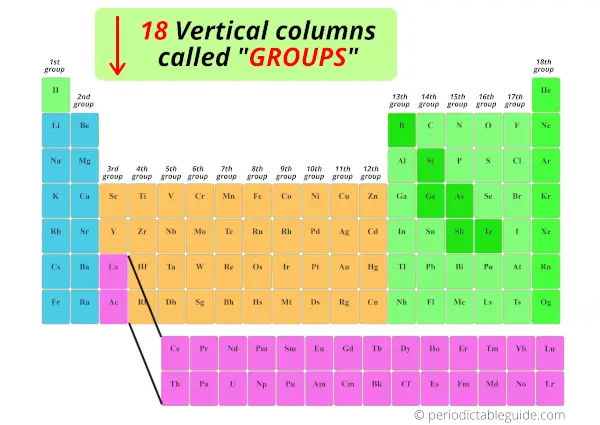
There are full 18 vertical columns in modernistic periodic table. (In other words, there are 18 groups in modern periodic tabular array)
Now, I'll tell you amazing things about Periods of the Periodic table.
We take seen that the horizontal rows of periodic table are known every bit periods.
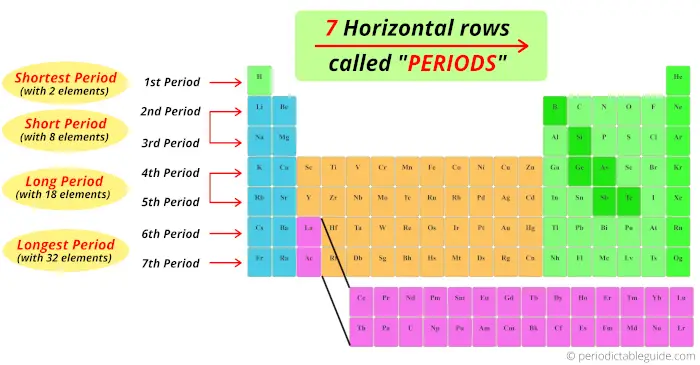
There are total seven horizontal rows in periodic table. That means there are total seven periods in periodic table.
At present, you can see that there are only 2 elements in the 1st catamenia. Thus, menses 1 is known as shortest catamenia.
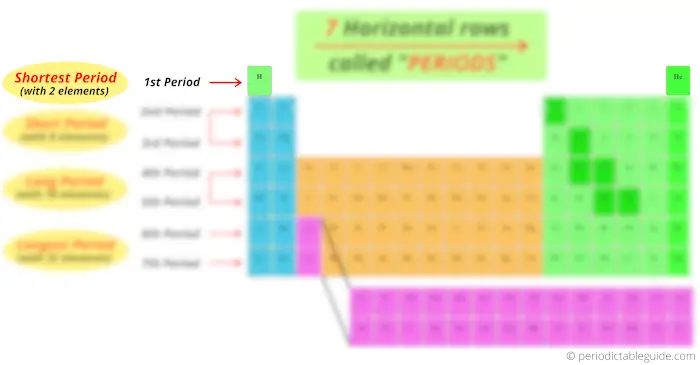
Now, the 2d catamenia and third period has viii elements, so period 2 and period 3 are known as brusk catamenia.
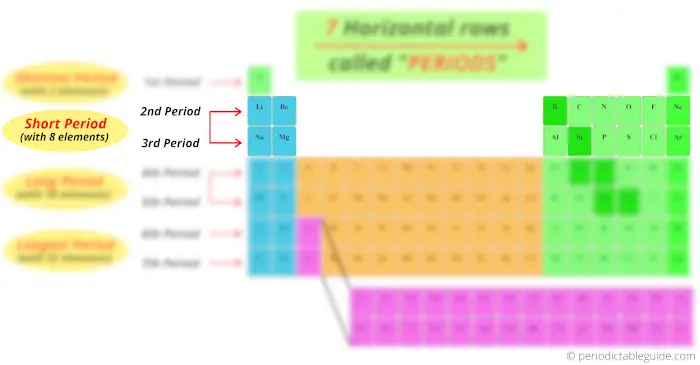
quaternary and fifth menstruum has total 18 elements. And then there are known as long period.
Now pay attention to this.
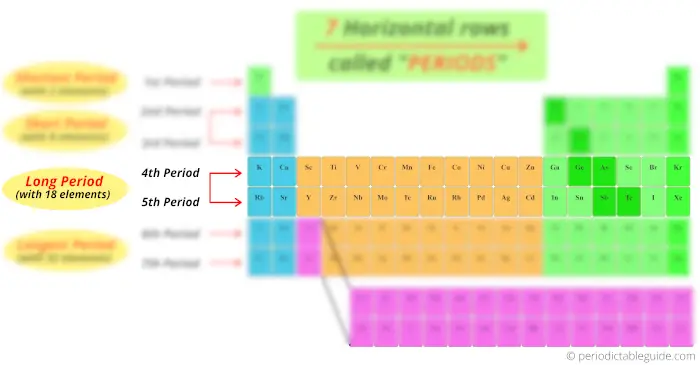
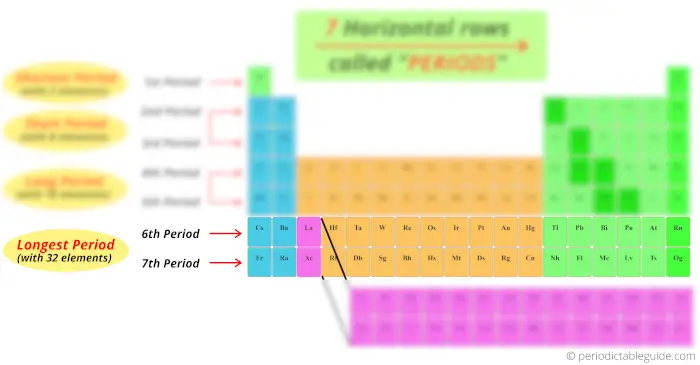
Look at the 6th and 7th menstruation, how many elements are there?
There are 18 elements + 14 elements at the bottom block. So full 32 elements are present in period vi and period 7.
Thus the 6th period and 7th period are known every bit longest period.
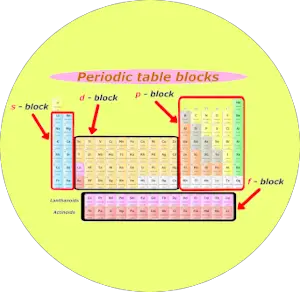
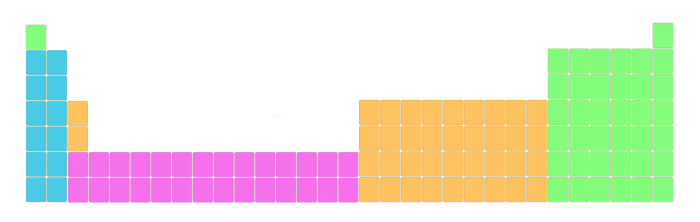
2). Blocks of periodic table
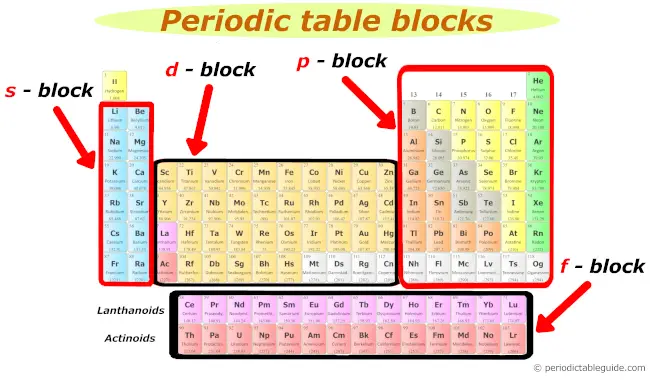
Well, these are the 4 blocks of periodic table.
- s cake
- p block
- d block
- f block
Let me give yous some overview of these iv blocks one past ane (in brief)
s block
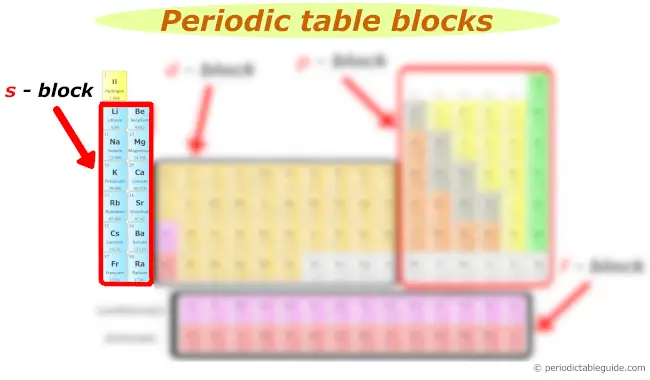
You can see the s block elements of the Periodic table in the above image.
s cake includes the following groups:
- Group 1 and
- Group 2
List of grouping i elements with their symbol are given below:
| Element name | Symbol |
| Lithium | Li |
| Sodium | Na |
| Potassium | K |
| Rubidium | Rb |
| Cesium | Cs |
| Francium | Fr |
List of grouping 2 elements with theirs symbols are given below:
| Chemical element proper noun | Symbol |
| Glucinium | Be |
| Magnesium | Mg |
| Calcium | Ca |
| Strontium | Sr |
| Barium | Ba |
| Radium | Ra |
Too read: Why are these elements known equally southward-block elements?
p cake
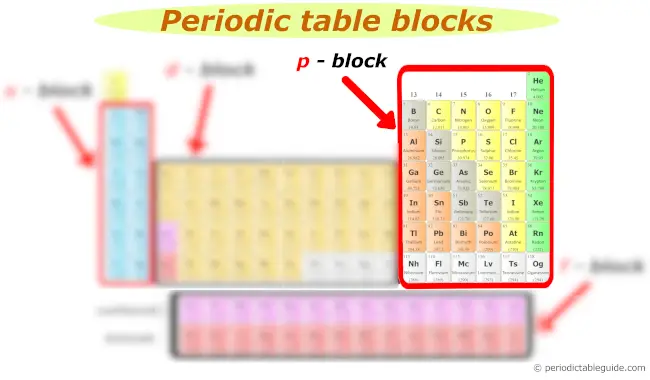
Now, here is your p block on the Periodic table.
The following groups are included in the p cake of periodic tabular array.
- Group 13
- Group xiv
- Group 15
- Group 16
- Group 17
- Grouping 18
List of grouping 13 elements with their symbol are given below:
| Chemical element name | Symbol |
| Boron | B |
| Aluminum | Al |
| Gallium | Ga |
| Indium | In |
| Thallium | Tl |
| Nihonium | Nh |
List of group 14 elements with their symbol are given below:
| Chemical element proper noun | Symbol |
| Carbon | C |
| Silicon | Si |
| Germanium | Ge |
| Tin | Sn |
| Led | Pb |
| Flerovium | Fl |
List of group 15 elements with their symbol are given below:
| Element name | Symbol |
| Nitrogen | N |
| Phosphorus | P |
| Arsenic | As |
| Antimony | Sb |
| Bismuth | Bi |
| Moscovium | Mc |
Listing of grouping 16 elements with their symbol are given below:
| Element name | Symbol |
| Oxygen | O |
| Sulphur | S |
| Selenium | Se |
| Tellurium | Te |
| Polonium | Po |
| Livermorium | Lv |
List of grouping 17 elements with their symbol are given beneath:
| Element proper noun | Symbol |
| Fluorine | F |
| Chlorine | Cl |
| Bromine | Br |
| Iodine | I |
| Astatine | At |
| Tennessine | Ts |
List of group eighteen elements with their symbol are given below:
| Chemical element name | Symbol |
| Neon | Ne |
| Argon | Ar |
| Krypton | Kr |
| Xenon | Xe |
| Radon | Rn |
| Oganesson | Og |
Also read: Why are these elements known equally p-cake elements?
d cake
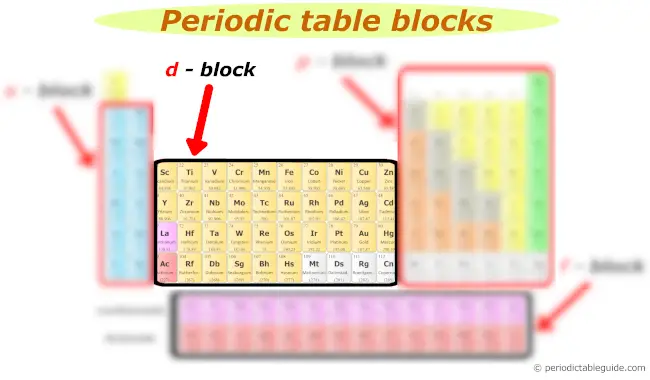
d block includes following groups.
- Group 3
- Grouping four
- Grouping 5
- Group half-dozen
- Group 7
- Group 8
- Grouping 9
- Group 10
- Grouping 11
- Grouping 12
The d block elements are as well known as Transition elements.
List of d block elements with names is provided in the below table.
| tertiary Grouping | 4th Grouping | fifth Group | 6th Group | 7th Group | 8th Group | ninth Group | 10th Group | 11th Group | 12th Group |
| Scandium (Sc) | Titanium (Ti) | Vanadium (V) | Chromium (Cr) | Manganese (Mn) | Atomic number 26 (Fe) | Cobalt (Co) | Nickel (Ni) | Copper (Cu) | Zinc (Zn) |
| Yttrium(Y) | Zirconium (Zr) | Niobium (Nb) | Molybdenum (Mo) | Technetium (Tc) | Ruthenium (Ru) | Rhodium (Rh) | Palladium (Pd) | Silverish (Ag) | Cadmium (Cd) |
| Lanthanum (La) | Hafnium (Hf) | Tantalum (Ta) | Tungsten (West) | Rhenium (Re) | Osmium (Os) | Iridium (Ir) | Platinum (Pt) | Gold (Au) | Mercury (Hg) |
| Actinium (Ac) | Rutherfordium (Rf) | Dubnium (Db) | Seaborghium (Sg) | Bohrium (Bh) | Hassium (Hs) | Meitnerium (Mt) | Darmstadtium (Ds) | Roentgenium (Rg) | Copernicium (Cn) |
Too read: Why are these elements known as d-block elements?
f block
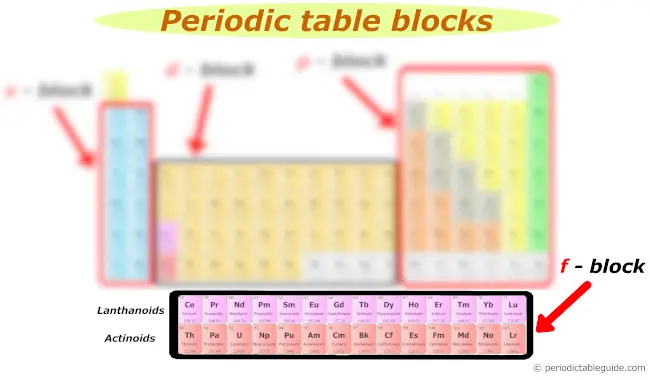
Many of yous might have a question;
"What are the two rows at the lesser of the periodic table?"
They are known every bit Lanthanides and Actinides.
The first row of elements in the f block is known as Lanthanides and the second row of elements in the f block is known as Actinides.
The f cake elements are also known as inner transition elements.
Also read: Why are these elements known as f-block elements?
iii). Types of elements on periodic table
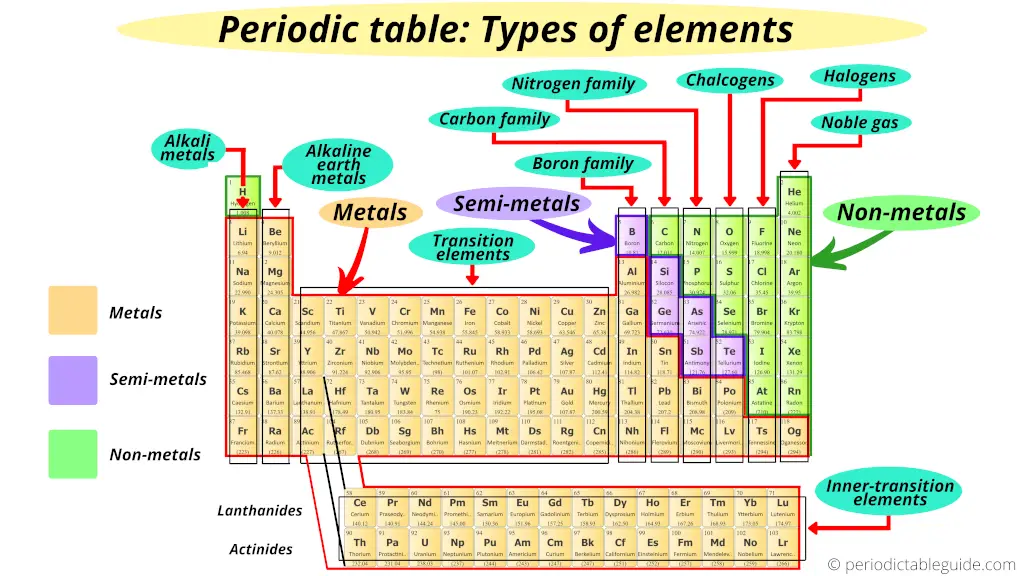
Now, I'll show y'all what type of elements are there on the Periodic tabular array.
You might have a question;
"Where are metals on the Periodic tabular array?"
"Where are nonmetals located on the Periodic table?"
"Where are metalloids located on the Periodic table?"
"Where are halogens on the Periodic table?"
"Where are Noble gases on periodic table?"
And many more…
Where are metals on the Periodic table?
Await, first of all you lot should know some backdrop of metal, nonmetal and semimetal (metalloid).
Just you lot volition empathise this position of elements even if y'all do not know anything. I'll try to explain this in a simple way.
But retrieve that, on the left side of the Periodic tabular array in that location are the all-time metals.
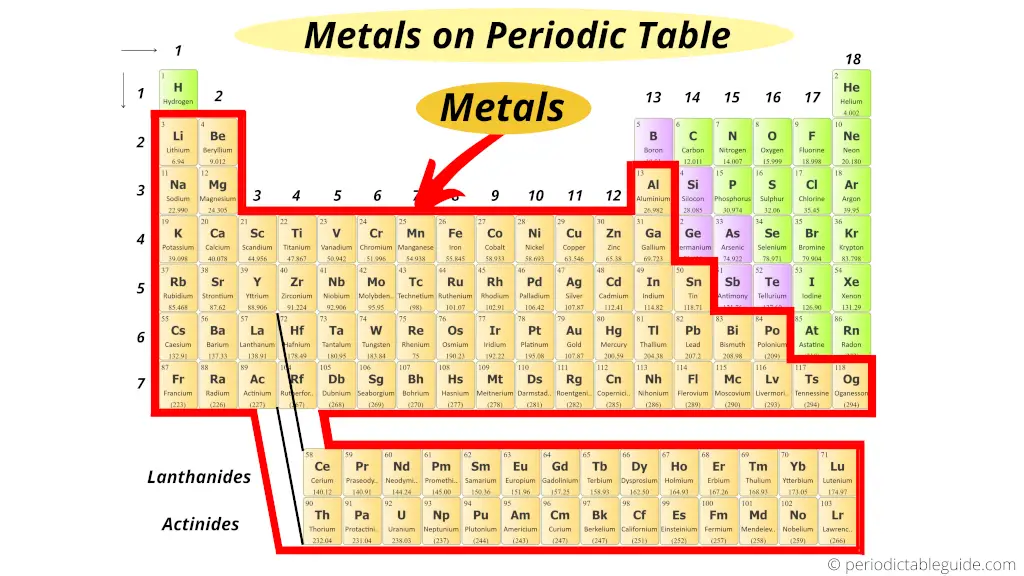
Further, co-ordinate to their availability, they are again classified equally Alkali metals and Alkaline Earth metals.
Also see:
i). How many metals are on periodic tabular array?
ii). Why are metals on the left side of periodic table?
Brine metals:
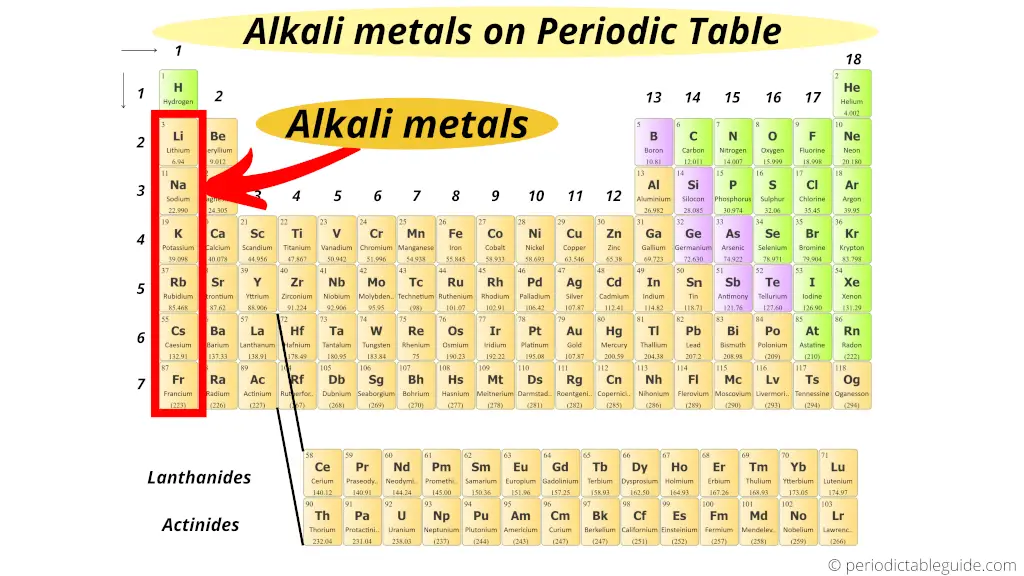
Grouping ane elements are known equally alkali metals.
All the elements of group one form an alkaline solution (or basic solution) when they react with h2o. (In other words, when they react with water, they form the compounds which are basic in nature)
That'southward why these grouping 1 elements are known equally alkali metals.
Also see:
i). Why are alkali metals and then reactive?
2). Electron configuration of brine metals
Alkaline Globe metals:
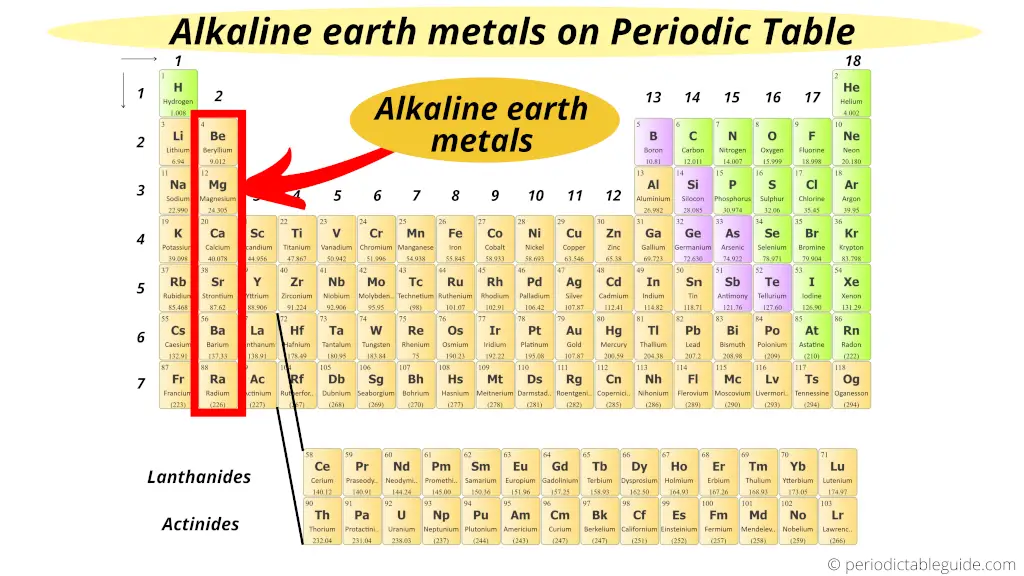
Group 2 elements are known as Alkaline Globe metals.
All these elements of grouping 2 have the similar properties as that of group 1 elements, just these elements are more often than not found from the Globe crust. Hence, they are named Alkali metal Globe metals.
Also run across:
1). What do alkaline earth metals accept in common?
2). List of alkaline earth metals with electron configuration
Where are nonmetals on the Periodic table?
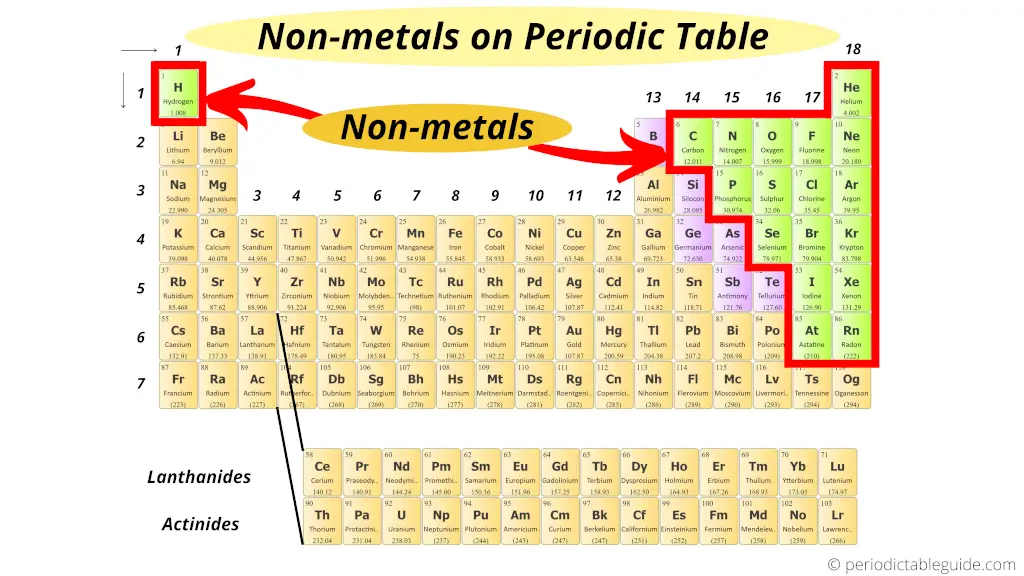
Here are the nonmetals on the Periodic table.
Read more about Nonmetals from hither.
Where are metalloids (semimetals) on the Periodic table?
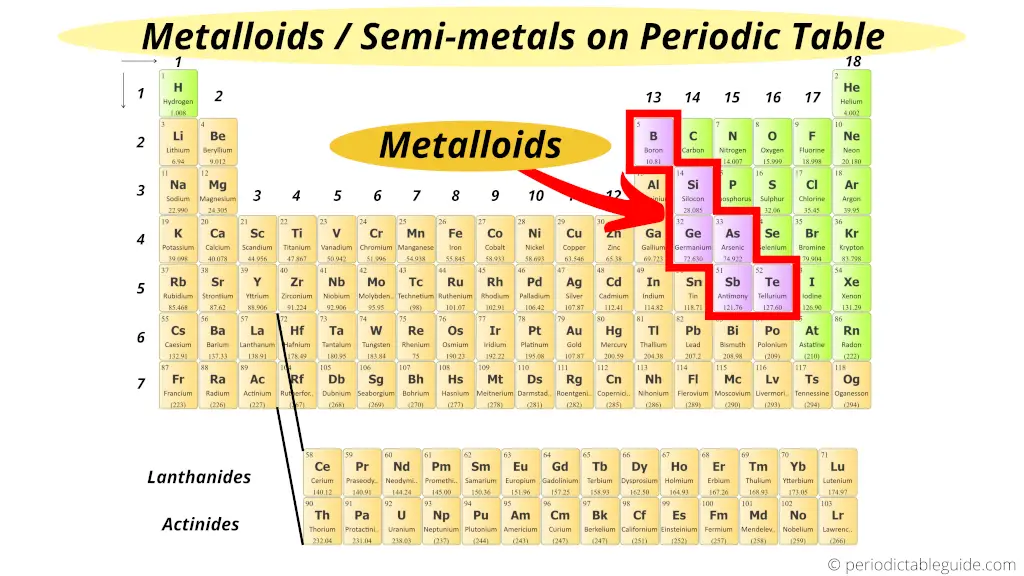
Here are the semimetals or metalloids on the Periodic table.
The zig zag line shown in the Periodic table includes the elements which possess metallic nature also as nonmetallic nature.
Thus they are known as semimetals or metalloids.
Too see: Physical and chemical properties of metalloids.
Where are halogens on the Periodic table?
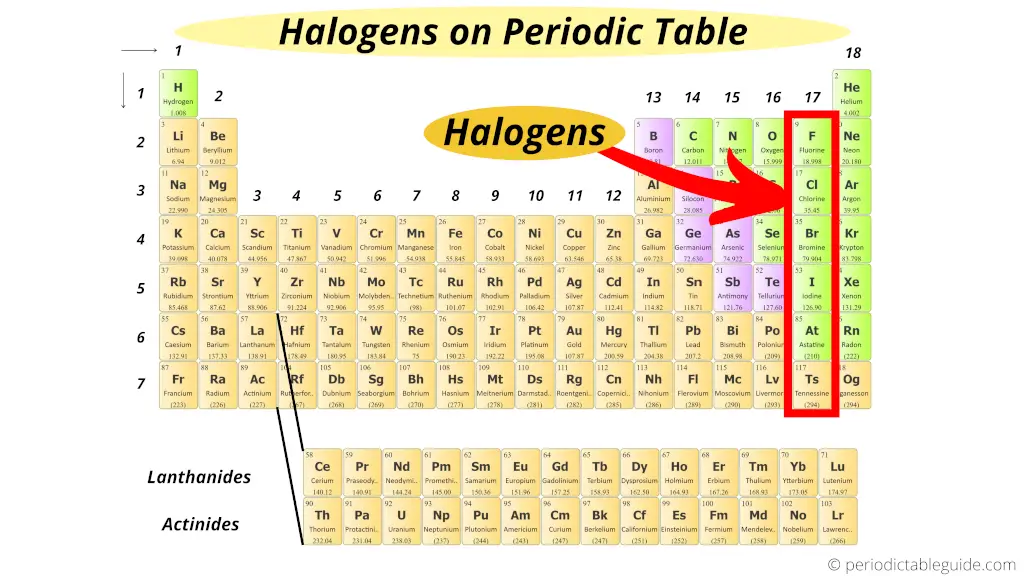
Do you know why halogens are called so?
The word Halogens has come up from two Greek words;
"Hal" meaning "salts" and
"Gen" meaning "to produce".
Thus "Halogens" means "salt producing".
So all the elements of the group 17 form salts when they react with metals.
For instance;
2Na + Cltwo ——-> 2NaCl
Which is a table salt.
Thus, group 17 elements are known equally Halogens.
Also see:
i). Which is most reactive halogen?
2). Properties of halogens.
Where are Noble gases on periodic table?
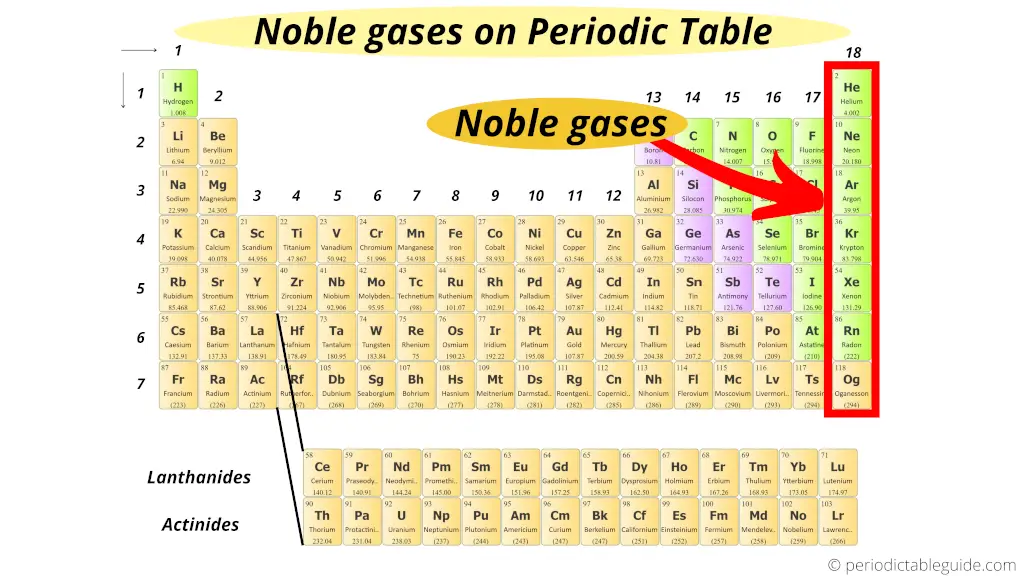
The elements of group 18 do not participate in whatever chemical reaction. (In other words, these elements are chemically inert)
Plus all the elements of grouping xviii are in gaseous state.
Thus, the 18th group elements of periodic table are known equally Noble gases or inert gases.
As well run into:
1). Electron configuration of noble gases
2). Uses of noble gases in daily life
Where are transition elements on periodic table?
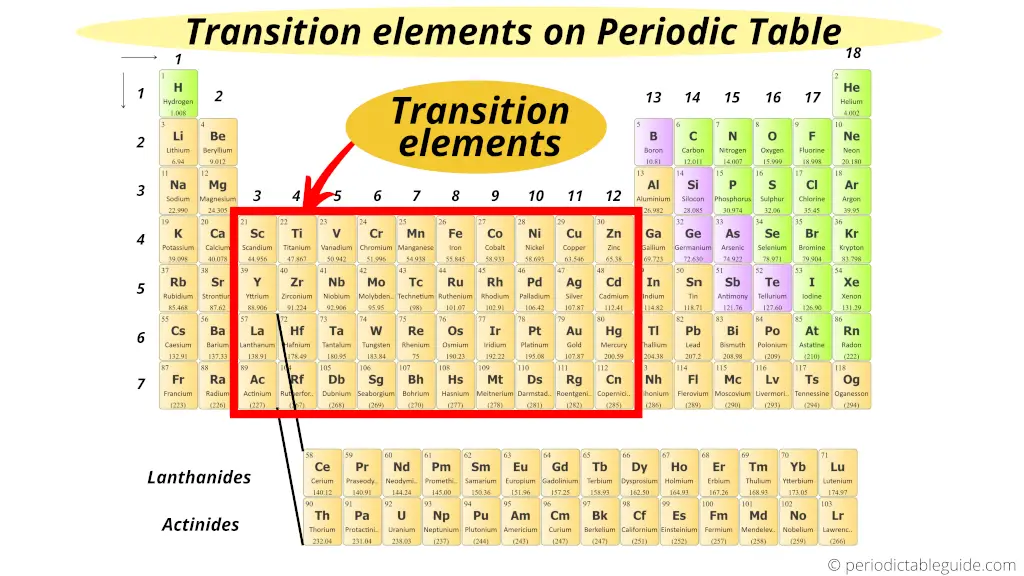
Transition metals (also known as Transition metals) form a bridge between the chemically active metals of south-block elements and the less active elements of Groups xiii and fourteen.
Thus these d cake elements are known as "Transition Elements".
Besides encounter: List + Electron configuration of transition metals.
Where are inner transition elements on periodic tabular array?
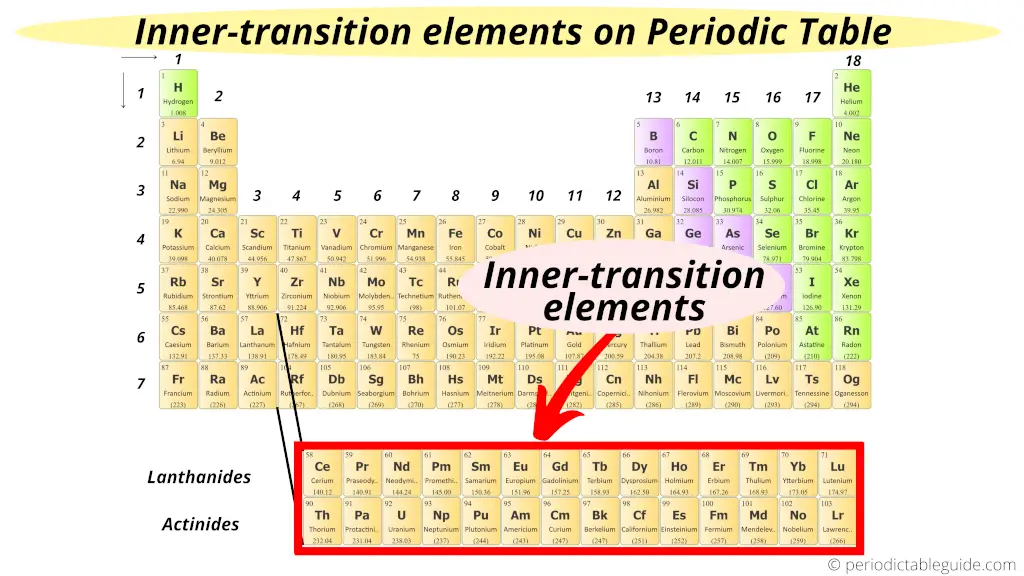
Now, this is very elementary.
As the name advise "inner". Means these elements are transition elements only but they are placed in the inner section of transition elements.
Hence these f block elements are known as "Inner transition elements."
Besides encounter: Why are inner transition metals at the bottom of periodic tabular array?
Where is boron family in the periodic tabular array?
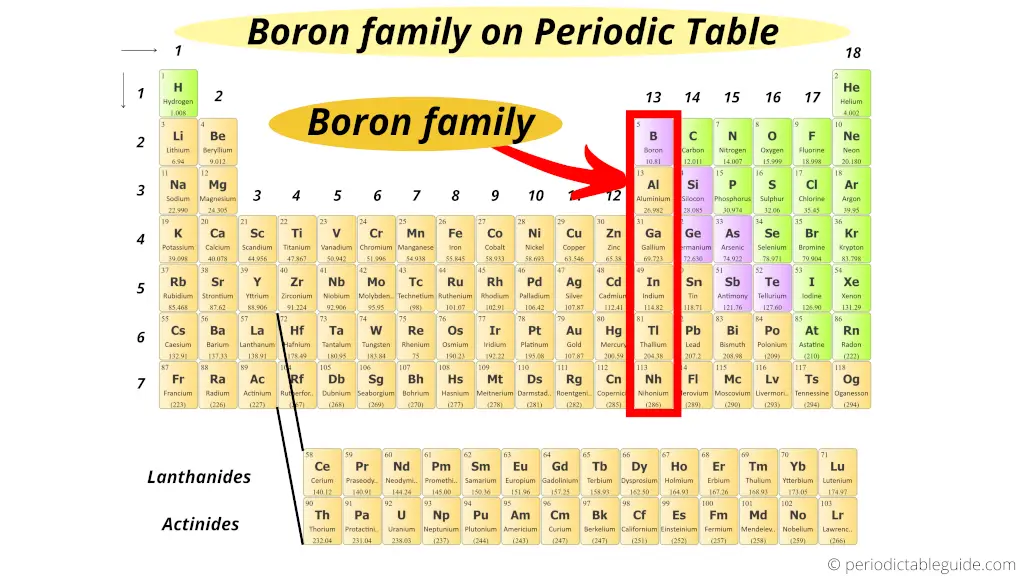
Very simple!!
The first element of group 13 is boron.
Hence, group thirteen is known every bit boron family or boron group.
Where is carbon group in periodic table?
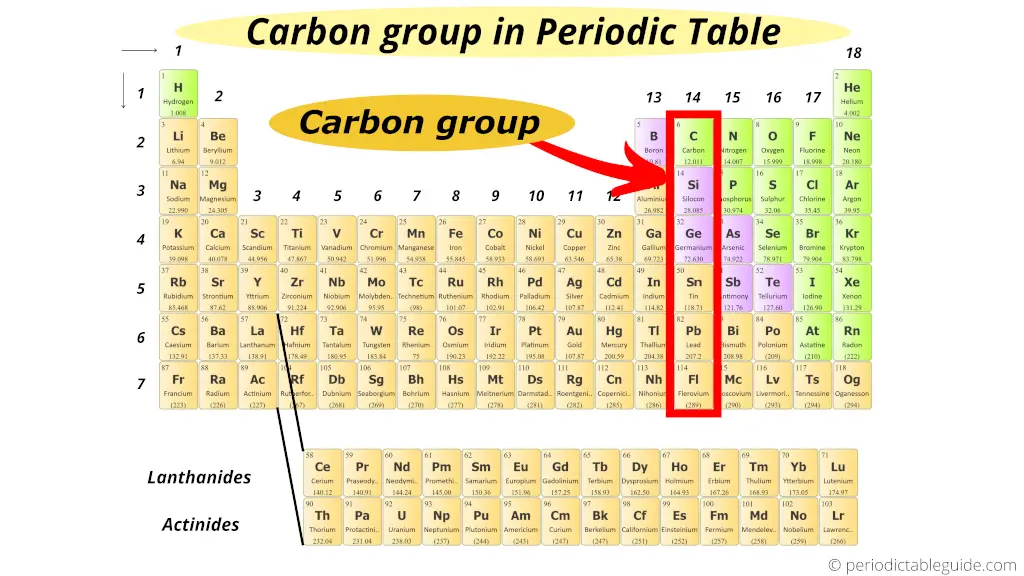
This is also elementary!!!
The starting time element of group fourteen is carbon.
Hence, group 14 is known as carbon family or carbon group.
Where is nitrogen family on periodic table?
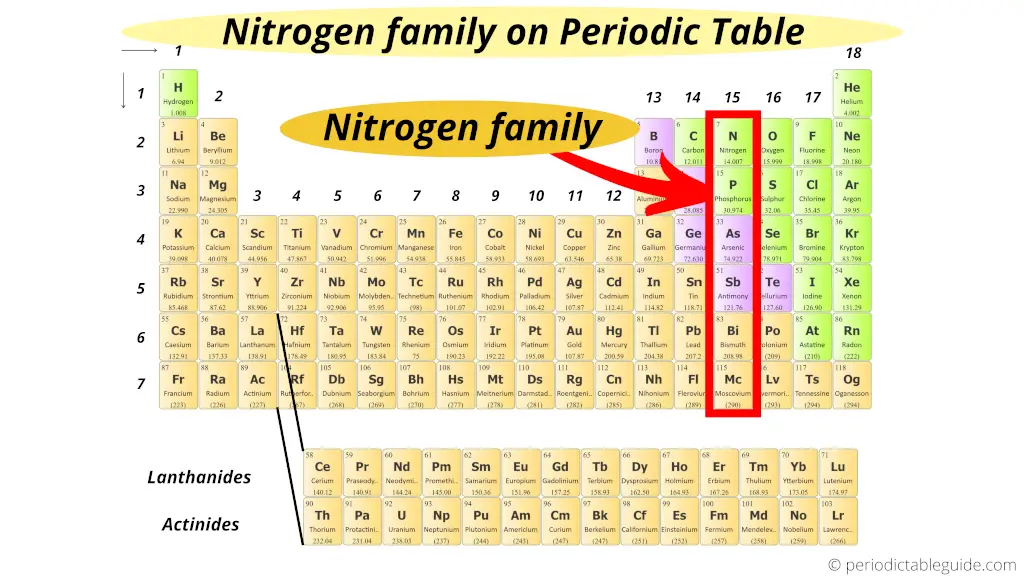
Group fifteen elements has the start element "Nitrogen".
And so the grouping xv is known as nitrogen family or nitrogen grouping.
These 15 group elements are also known as Pnictogens.
Where are chalcogens on periodic table?
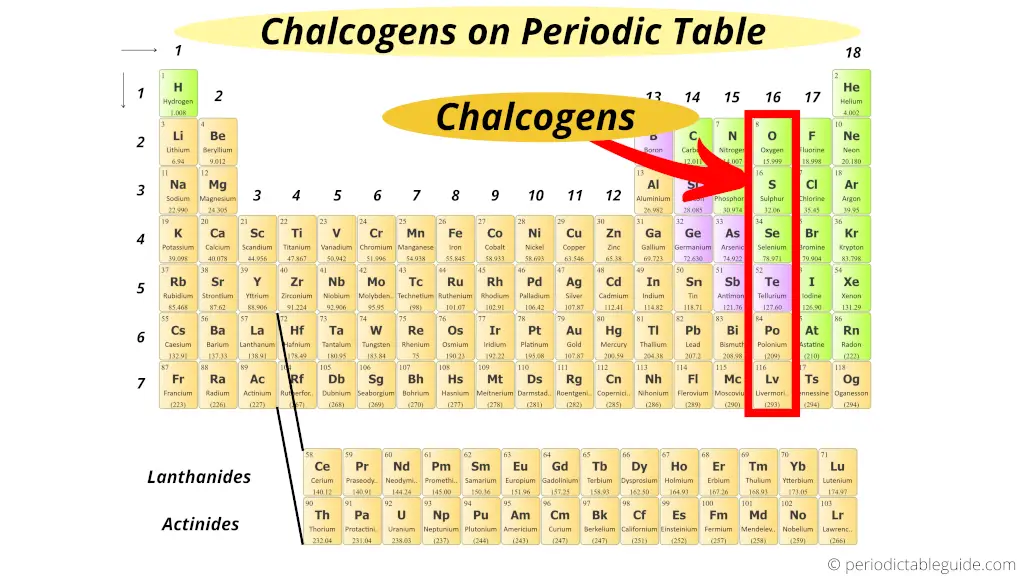
Group xvi elements includes oxygen, sulphur, selenium, tellurium and polonium.
These elements are by and large present in the World chaff in the course of ores.
Chalcogens is the Greek word which means "ore forming".
As these grouping 16 elements are present in the ores, they are known as "Chalcogens."
Enjoying?
If no, so also
Keep reading…. Because the about important part is coming now.
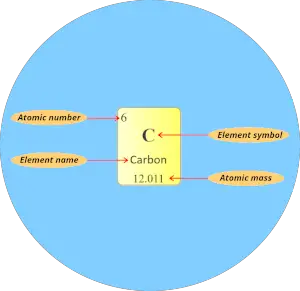
How to read periodic table elements?
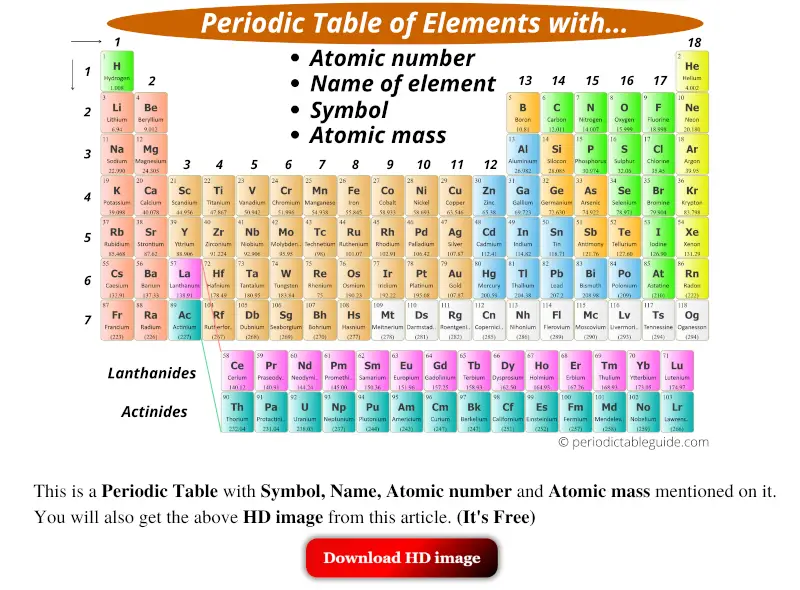
Here is your sweetness, cute, beautiful, colorful mod periodic table. Hehehe…
At present, you might be wondering;
- What is written in each single square box?
- What are those 1, two, 3, 4, etc… numbers?
- What is that number written beneath each chemical element?
- Why do the columns have different colors?
- What are those ii rows at the bottom of the periodic table?
- How to recollect periodic table?
- And many more…
Do not worry at all, I will explicate everything step by step.
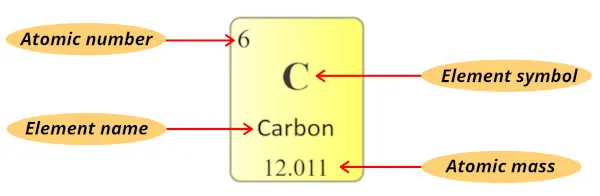
I will give you lot a very short and simple explanation for reading the elements of periodic tabular array using the instance of carbon element.
Atomic number:
The total number of protons present in the nucleus of an atom is known as Atomic number. Or
The number of electrons possessed by a neutral atom is called Diminutive number.
Here, the number written on the top of each square is the atomic number of that chemical element.
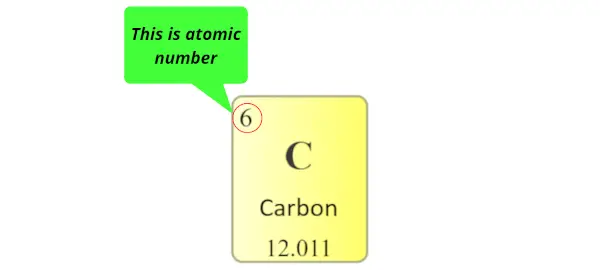
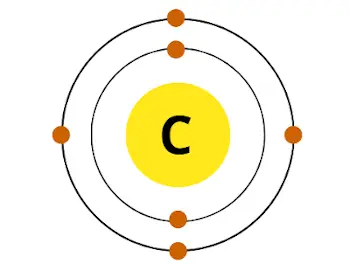
For example carbon has total 6 electrons. So the diminutive number of carbon is 6.
Element symbol:
The alphabet used to mention the elements is known as the symbol of elements.
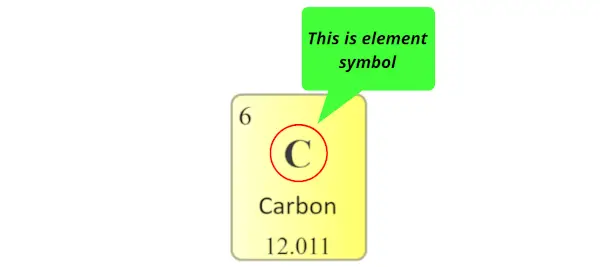
The symbol of elements are represented by using unmarried capital letter alphabet (for e.grand. C, O, N, F, H, etc…) or sometimes outset letter is majuscule followed by lowercase alphabet (for e.grand. Li, Na, Si, Ne, Ar, etc…)
Element name:
The proper noun displayed in each individual square block represents the proper name of that chemical element.
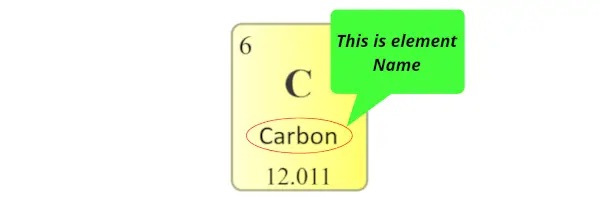
Diminutive mass:
Atomic mass is the sum of the masses of protons and neutrons present in the nucleus of an cantlet.
Diminutive mass in periodic table is displayed as shown in beneath epitome. (It is mostly a decimal value)
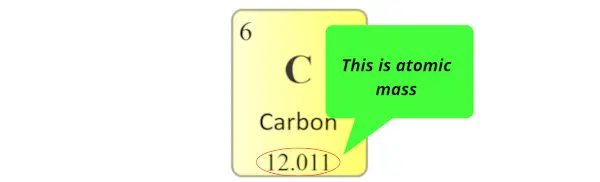
(Notation: In some periodic tables, you may find that the atomic masses are written in the left top corner or right meridian corner of each prison cell, merely recall that it is a decimal value. Then if you observe that decimal value in the cells of periodic table, it'due south an atomic mass of that element)
Now the most important topic is coming…
If yous are yet with me, then Yous are going to Rock now.
Keep reading…
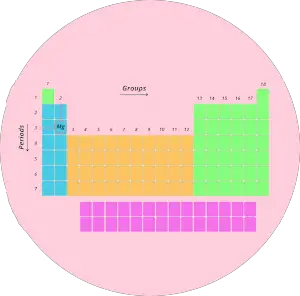
What does group number and period number represent on the periodic tabular array?

Trust me this is very simple.
Permit me explain straight with an example.
Example one:
Let's have an example of magnesium (Mg)
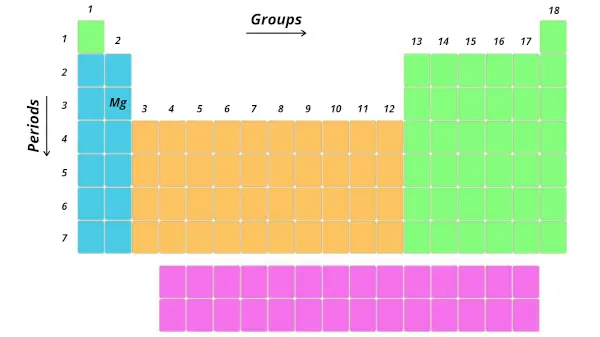
Now, tell me where is the position of magnesium (Mg).
It is in third period and 2d group. Right?
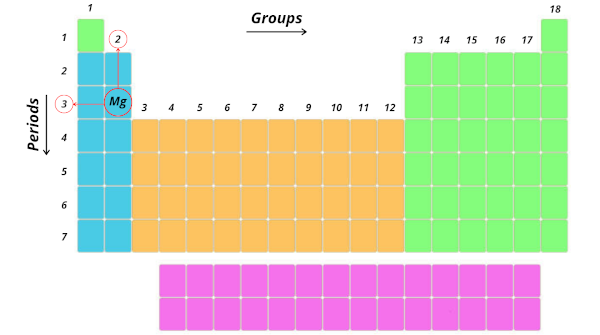
Cool…Now, this period 3 indicates that the atoms of magnesium possess 3 orbits or 3 shells.
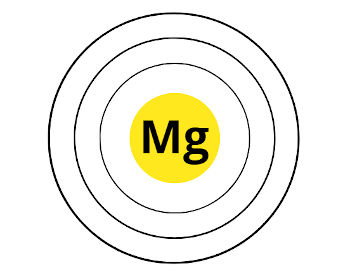
Here are the 3 orbits of magnesium.
Now, Magnesium (Mg) is lying in group ii. That means, information technology has 2 electrons in its outermost orbit.
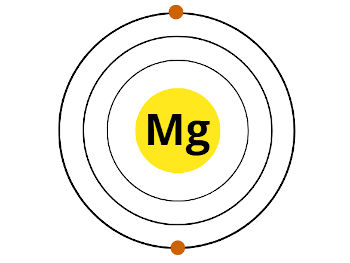
Hither are the 2 electrons in the outermost orbit of magnesium. These two electrons are known as valence electrons.
And you might be knowing that each orbit has some different capacity to concur electrons.
See the table beneath, you will come to know "how many electrons a particular orbit can concord."
Number of electrons in shells:
| Orbit / Vanquish (n) | Maximum no. of electrons this orbit can hold |
| K crush, n = one | 2 × one² = two |
| L beat out, n = 2 | 2 × two² = viii |
| K trounce, due north = 3 | 2 × iii² = 18 |
| N shell, north = 4 | two × 4² = 32 |
Thus,
- 1st shell tin can hold 2 electrons.
- 2nd shell tin hold 8 electrons.
- 3rd shell can agree 18 electrons.
- fourth shell can hold 32 electrons.
Let's come dorsum to magnesium. It is having diminutive number 12. That means information technology has total 12 electrons.
From the above table, nosotros tin say that, first orbit will hold 2 electrons, the second orbit will concord viii electrons and finally remaining 2 electrons will be in the 3rd orbit.
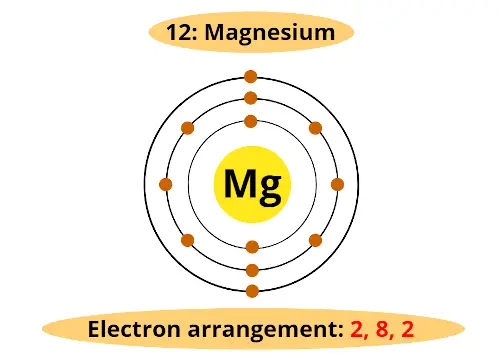
(Side notation: The actual orbits are non 2 dimensional, merely they are iii dimensional. In order to represent on the newspaper, nosotros draw two dimensional orbit)
Instance ii:
Let'south take some other instance of Chlorine (Cl).
Chlorine lies in the 3rd period and 17th group.
3rd menstruation indicates that chlorine has 3 orbits and;
17th grouping indicates that it has 7 electrons in outermost orbit.
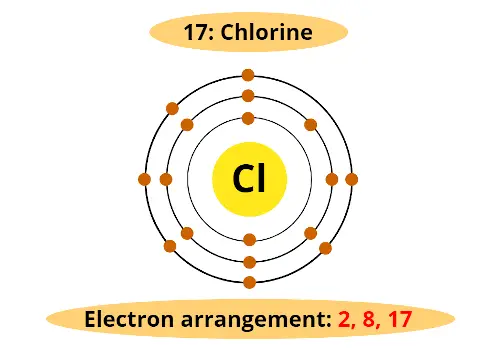
Atomic number of chlorine is 17. (In other words, Chlorine has full 17 electrons).
And now you know very well that 2nd orbit holds maximum viii electrons.
Thus, the arrangement of electrons in the orbit of Chlorine cantlet is as shown to a higher place.
Recollect:
Grouping 13 indicates three valence electrons, not 13.
Group fourteen indicates 4 valence electrons, non xiv.
Grouping 15 indicates five valence electrons, not 15.
Group 16 indicates 6 valence electrons, non 16.
Group 17 indicates vii valence electrons, not 17.
Group 18 indicates 8 valence electrons, not xviii.
I accept explained the concept of valence electrons for d block and f block elements in separate articles "d block elements" and "f block elements." (Because information technology is piddling chip different and commencement you need to know near the concept of orbitals)
Are you all the same with me…?
Then let'due south non waste more than time.
A most of import and astonishing concept about the periodic table is coming…
Trends in periodic table (Periodic trends)
I'll keep this very short and to the indicate. (Because this commodity is very long now). If you lot want detailed information on this topic, you must visit: Main article on Periodic trends.
Allow's dive direct into information technology.
First of all, what does trends mean in Periodic tabular array?
Trends: The change in properties of elements down the groups (from summit to bottom) and across the periods (from left to right) is known equally trends.
We generally measure these trends (modify in properties of elements) downwardly the group and beyond the period.
I'll shortly tell you the variation of following properties in the periodic table.
- Valency tendency
- Diminutive size tendency
- Metallic grapheme trend
- Non metallic character trend
- Electronegativity trend
- Electron affinity trend
- Ionization energy trend
Periodic trends: Valency
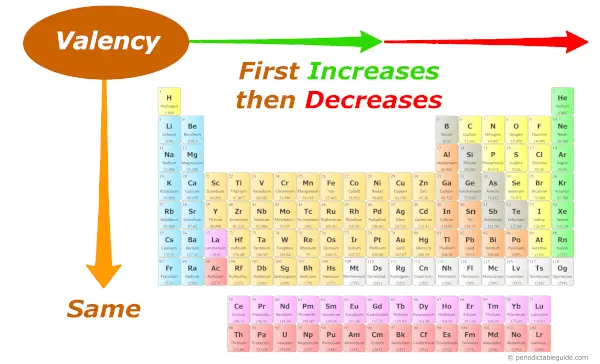
As nosotros move across the period (from left to right), the Valency of the elements outset increases and and so decreases. While moving down the group (from superlative to bottom), the Valency of elements remains the same.
Don't worry, I'll tell you the reason why this happens.
See, beginning of all valency is nothing but the number of electrons required to consummate the octet.
For example, atomic number of oxygen is 8. That means oxygen has total 8 electrons.
Then information technology'southward electron arrangement is (2, 6).
It has two electrons in first orbit and other 6 electrons in second orbit.
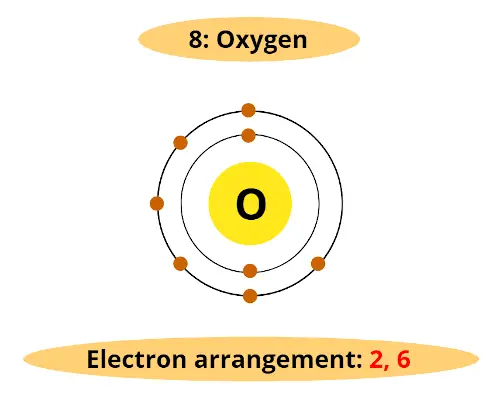
You can come across that oxygen has vi electrons in its outermost orbit.
Thus, it requires 2 electrons to complete the octet.
Hence valency of oxygen is two.
Every bit we move across the period (from left to correct), the Valency of elements first increases and so decreases.
And along the group, the Valency remains abiding, because you know very well that the number of electrons in the outermost orbit remains the same for all the elements of the same group.
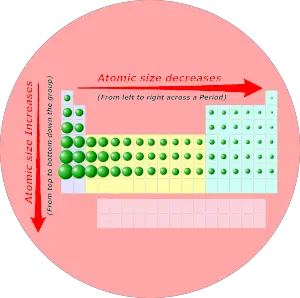
Periodic trends: Diminutive size
Every bit we motility across the catamenia (from left to correct), the atomic size of elements decreases. While moving downwards in the group (from top to lesser), the atomic size increases.
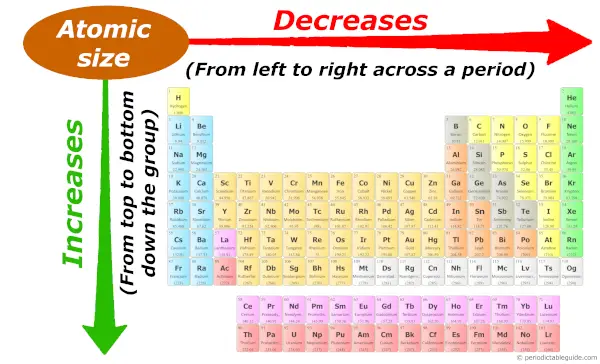
Desire to know why the atomic size decreases across a period and why it increases down the group?
And so bank check out the separate article on Atomic size trends . (Where I have explained the reason with images and example).
Periodic trends: Metallic character
Equally we motion beyond the menstruation (from left to right), the metallic character of elements decreases. While moving downwardly in the group (from top to bottom), the metallic character increases.
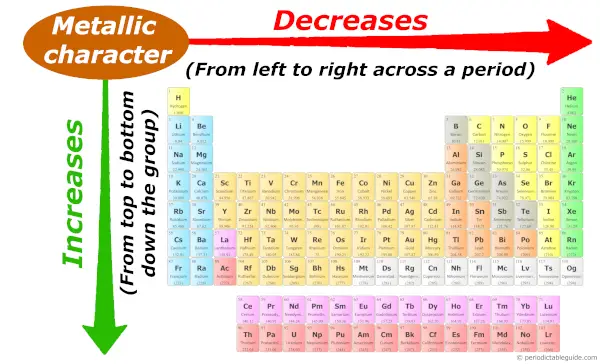
To know the reason behind this tendency, I highly recommend y'all to visit the split commodity: Metallic character trend in Periodic table.
Periodic trends: Non metallic character
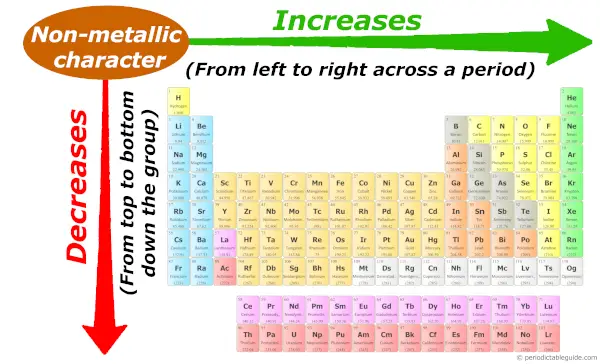
As nosotros motility across the period (from left to correct), the non metal grapheme of elements increases. While moving down in the group (from superlative to bottom), the non metallic character decreases.
I have given little more than data on non metallic graphic symbol trends. So if you want to run across it, and so kindly visit this article: Not metallic character trend in Periodic table.
Periodic trends: Electronegativity
Down the grouping (from elevation to bottom), the atomic size increases. So Electronegativity decreases.
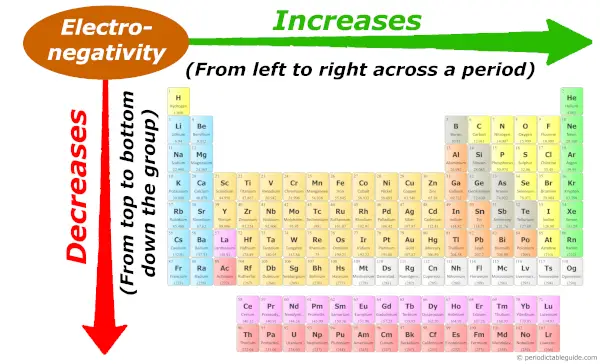
And beyond the period (from left to right), the atomic size decreases, so the Electronegativity increases.
Simply if you don't know what electronegativity is, then you tin refer to this article (where I accept explained the simple plain english language caption virtually electronegativity and few more things about the electronegativity trend in Periodic tabular array.)
Periodic trends: Electron affinity
As we motility across the period (from left to right), the atomic size decreases, so electron affinity increases across the catamenia (from left to right.)
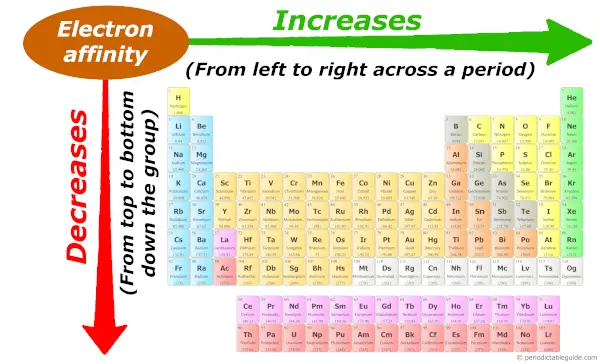
And as we move down the group (from summit to bottom), the atomic size increases, and then the electron analogousness decreases down the group (from meridian to bottom). Now, if you lot don't know about the concept of electron affinity, plus if yous want to know the reason why electron affinity increases beyond a period and why it decreases down the group, then visit this commodity: Electron analogousness trend in Periodic table.
Periodic trends: Ionization energy
Across the menses (from left to right), the diminutive size decreases, so ionization energy increases across the menses (from left to right.)
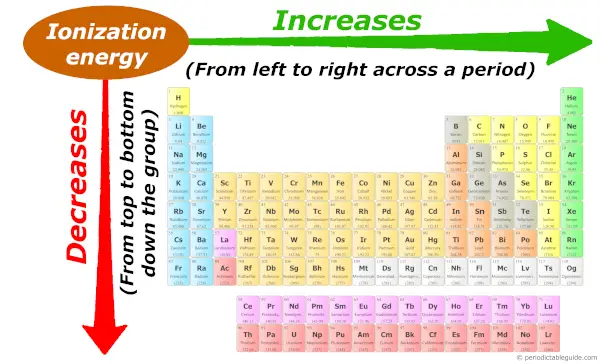
And down the group (from top to bottom), the atomic size increases, and so the ionization energy decreases down the grouping (from top to bottom.)
Desire to know why this trend is so?, then visit the Guide on Ionization energy trend , where I have explained the concept of ionization energy with images + I have besides explained the reason why this trend is so?
Summary of Periodic trends
So equally a summary, yous can remember all the trends in periodic tabular array with this single prototype.
Kudos…!!
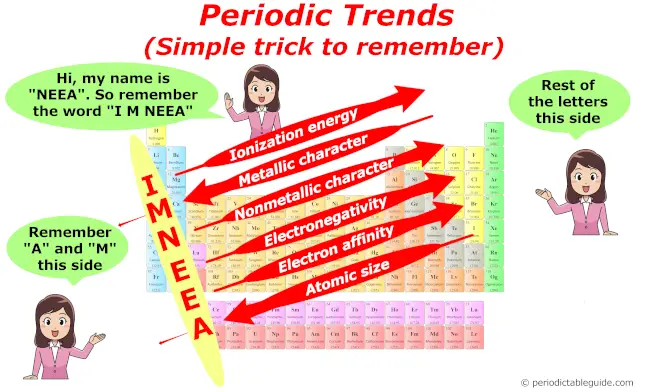
Source: https://periodictableguide.com/periodic-table-of-elements/
0 Response to "Periodic Table of Elements Easy to Read"
Post a Comment